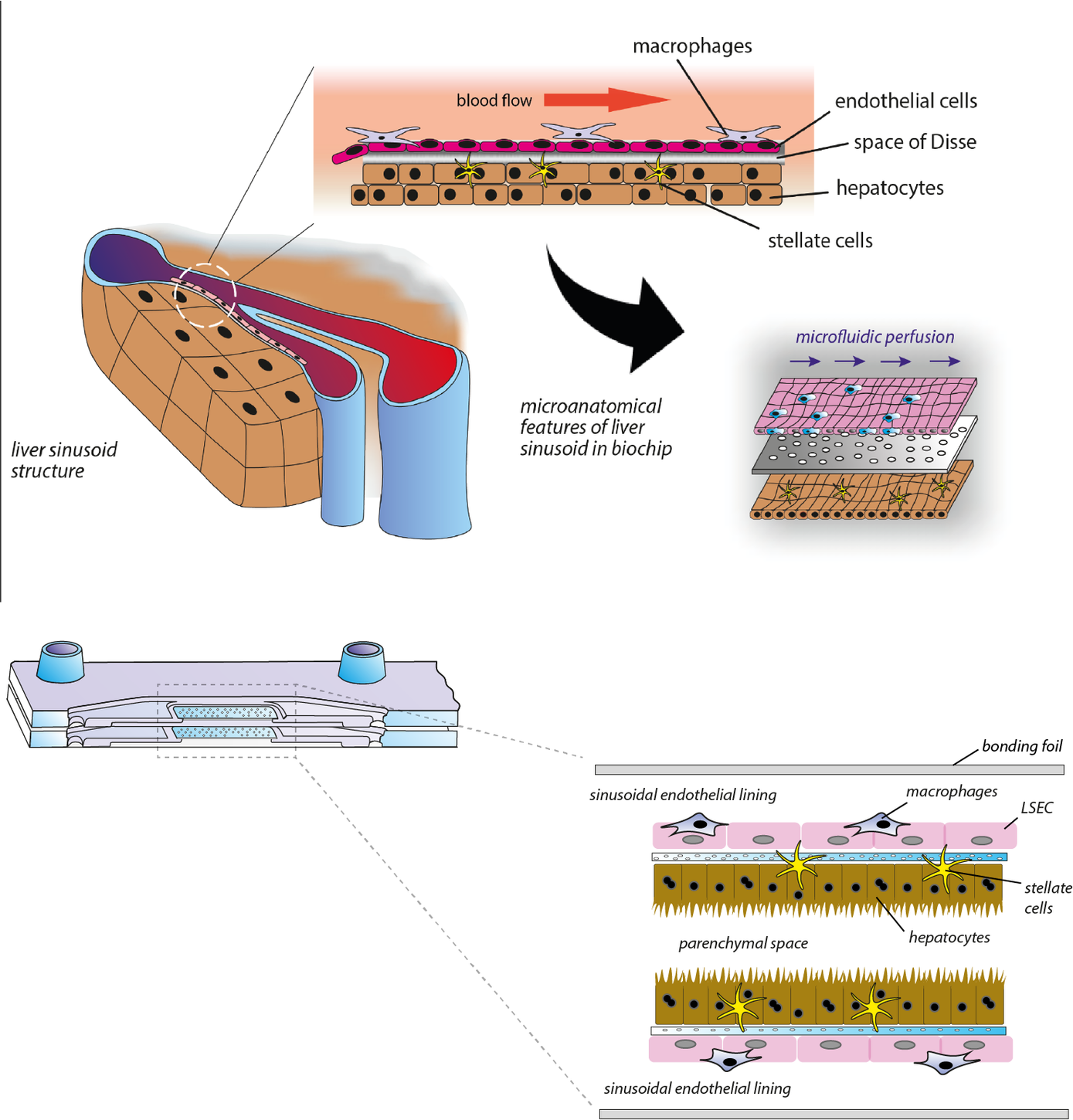
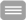The liver harbors about 80% of all macrophages of the human body. Circulating monocytes are constantly patrolling within the hepatic vascular system in search of pathogen-associated molecular substances and are able to migrate into the hepatic tissue after detection of pathogens. To avoid unwanted immune responses under physiological conditions endogenous microbial molecules derived from commensal microbiota are well tolerated by the liver. However, in case of blood borne infections a prompt and robust inflammatory response is required to prevent further dissemination of the pathogen. In this context Kupffer cells, tissue resident macrophages of the liver, are central players in orchestrating a balanced immune response between immunotolerance and inflammation.
To investigate the underlying cellular and molecular mechanisms of the adapted immune reactions we developed a microfluidically perfused liver-on-chip model. The organ model includes all essential cell types of the human liver and allows the emulation of an microphysiological environment recreating organotypic functions of the liver in vitro. In the model, both inflammation-associated molecular processes of hepatocellular dysfunction as well as mechanism of organ regeneration after acute liver inflammation could be studied. The liver-on-chip platform has been successfully used in studies on drug metabolism, development of novel drug delivery systems, and disease modelling of acute and chronic liver inflammation and liver infections.