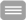Commensal microorganisms of the intestinal microbiome support the digestion and absorption of nutrition by the gut. Microbial colonization is supported by the host via a mucus layer secreted by epithelial cells organized in a complex tissue comprising villi and crypts that form a tight and protective barrier between the microbiota and the circulation. A physiological communication between the members of the intestinal microbiome and their host is crucial for the maintenance of homeostasis in the human body. Thus, deregulation and imbalance of these interactions known as dysbiosis are directly associated with the development of human diseases, including diabetes, obesity, inflammatory bowel disease (IBD), cancer, depression and non-infectious inflammatory diseases caused by opportunistic pathogens.
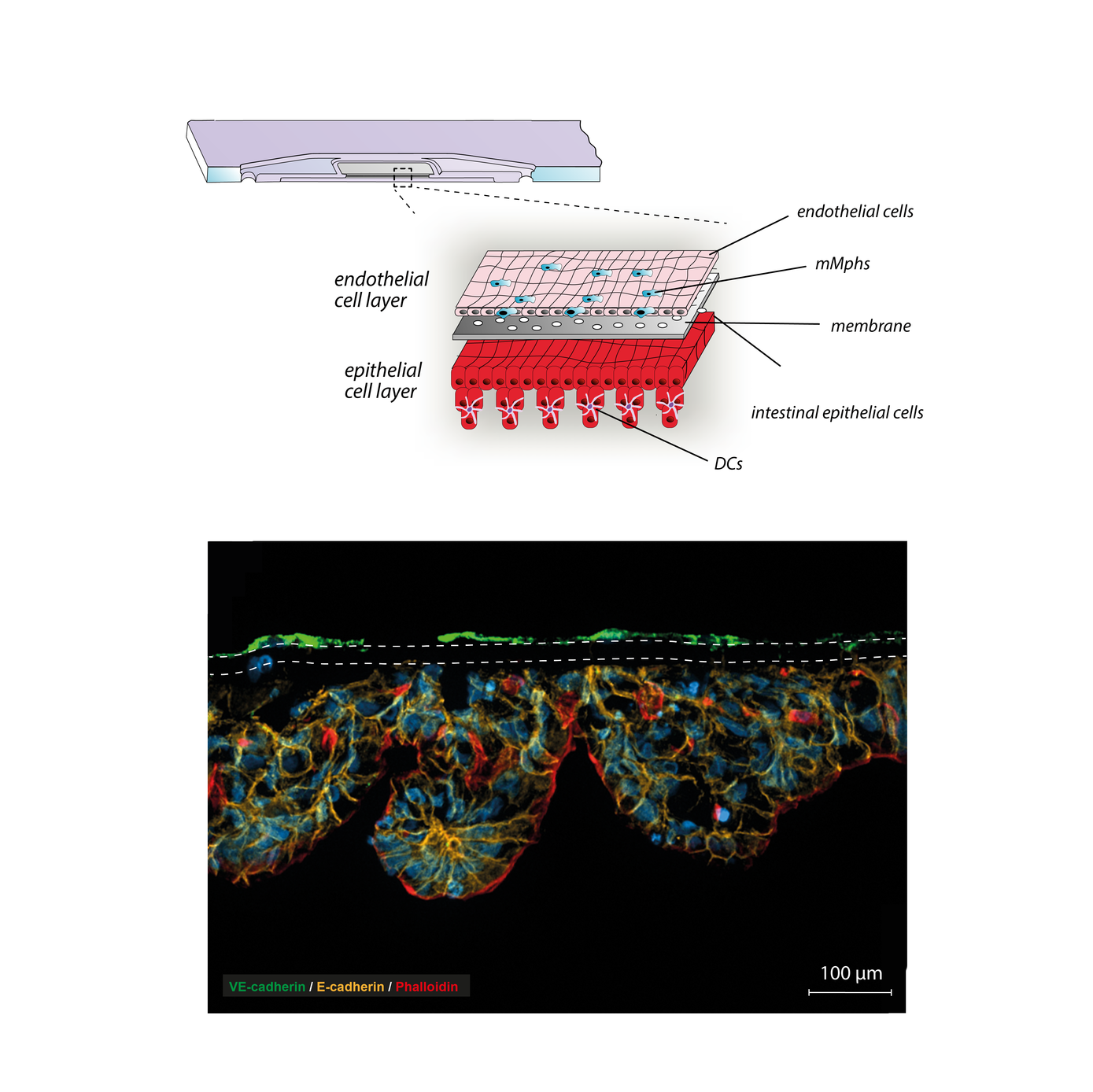
To create a platform for the investigation of the underlying mechanisms of dysbiosis associated diseases, we established a three-dimensional microphysiological model of the human intestine. This model resembles organotypic microanatomical structures and includes tissue resident innate immune cells exhibiting features of mucosal macrophages and dendritic cells. The model displays the physiological immune tolerance of the intestinal lumen to microbial-associated molecular patterns and can, therefore, be colonised with live microorganisms. It represents a valuable tool to systematically explore the underlying mechanisms of microbial communication, host-microbe interactions, microbial pathogenicity mechanisms, and immune cell activation under physiologically relevant conditions in vitro. Further, it allows the screening and development of novel treatment strategies for IBD including pharmaceutical treatments and adjustment of dysbiosis conditions to maintain physiological conditions of the human microbiota that keep opportunistic pathogens in their commensal state and prevents the onset of related inflammatory diseases.