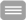In conventional fluorescence microscopy only the information conferred by the fluorescence intensity is exploited. But also other intrinsic properties of a fluorophore such as the fluorescence lifetime can be used to retrieve additional information about the sample. Fluorescence lifetime imaging (FLIM) can report on photophysical events that are too difficult (or impossible) to observe by fluorescence intensity measurements.
Since the lifetime of a fluorophore depends on its local environment it can be used as a sensor for properties of the solvent such as the viscosity and dissolved compounds such as ions or oxygen. By exploiting physical phenomena such as Förster energy transfer (FRET), FLIM can be used to test if molecules are in close vicinity to each other. In this way molecular interactions can be “imaged”.
In the lab of the Biomolecular Photonics Group several methods to measure fluorescence lifetimes are established. One technique is based on time-correlated single photon counting (TCSPC); the other method is based on a streak-camera system. This course will give an introduction into the technical equipment necessary to acquire the data and the mathematical algorithms used to analyze the data.
Further information about the course can be found on the website of the Biomolecular Photonics group: https://www.uniklinikum-jena.de/photonik/en/Teaching/Summer+School.html
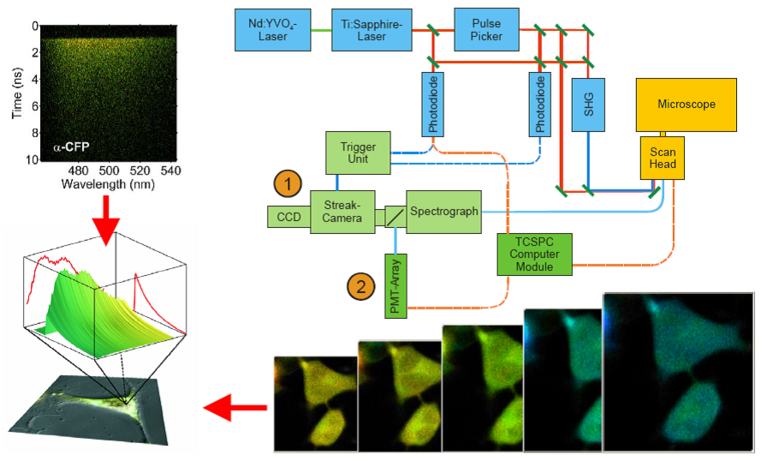
Setup used for fluorescence lifetime measurements. A pulsed Titanium:Sapphire (TiSa) laser or a supercontinuum laser are used for pulsed excitation of the biological sample on the stage of a confocal laser scanning microscope. Fluorescence emitted by the sample is recorded with a streak-camera (1) or a fast PMT-array (2) with a high spatial (x,y), temporal (t) and spectral (l) resolution.