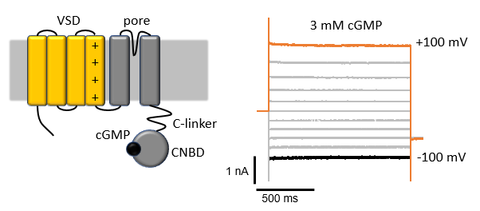
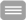Cyclic-nucleotide gated (CNG) channels, which couple intracellular cyclic nucleotide concentration with electric signaling in the retina and in the olfactory sensory cells, are a peculiarity within the family of voltage-gated cation channels. Even though they possess a perfectly conserved voltage sensor domain (VSD), they are virtually voltage independent.
In this project, identify the relevant amino acid sequences that disrupt the voltage dependence of CNG by using point mutations and chimeric channels of the human hCNGA1 and the voltage-gated hERG channel. The channels are expressed as homotetramers in Xenopus laevis oocytes and screened with the two-electrode voltage-clamp technique. Constructs with a significantly altered voltage dependence are further characterized by recording of gating currents. The final goal is a synthetic CNG channel with restored voltage dependence.