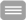In vivo MR spectroscopy (MRS) is currently one of the few methods allowing quantitation of biochemical markers (metabolites) in living organisms on human MR scanners. In terms of clinical applications, the relevance of MR spectroscopy is related to the fact that measurements are localized, non-invasive and are conducted without the use of ionizing radiation. Thus, the extracted metabolic markers can be evaluated by considering the tissue structure or functional parameters as they are assessed by MR imaging in order to gain insight into biochemical processes which accompany or even trigger morphological changes in diseases. Besides being able to conduct measurements in single voxels (eg in herds of disease, study volume ≥1 ml), MRS can be also combined with spatial encoding methods as they are known from MR imaging in order obtain 2- or 3-dimensional metabolite maps (Chemical Shift Imaging, CSI). Human applications typically comprise proton and phosphorus MRS scans (1H- and 31P-MRS), which are relying on the excitation of hydrogen and phosphorus nuclei of corresponding metabolites. Among other things, 1H-MRS can be used to access the density and integrity of different cell types (N-acetyl aspartate and myo-inositol in the brain) and cell membrane turnover markers (choline), which is particularly important for monitoring neurodegenerative and oncological diseases (see the upper chart in figure on the right). On the other hand, 31P-MRS offers the opportunity to quantify important energy metabolism markers such as ATP and phosphorus creatine as well as tissue pH value (see the lower chart in figure on the right). In general, the Medical Physics group is working on the development of new methods for acquisition, artifact suppression, processing and quantification of MR spectroscopic data from the brain and musculature.
MR Spektroskopie
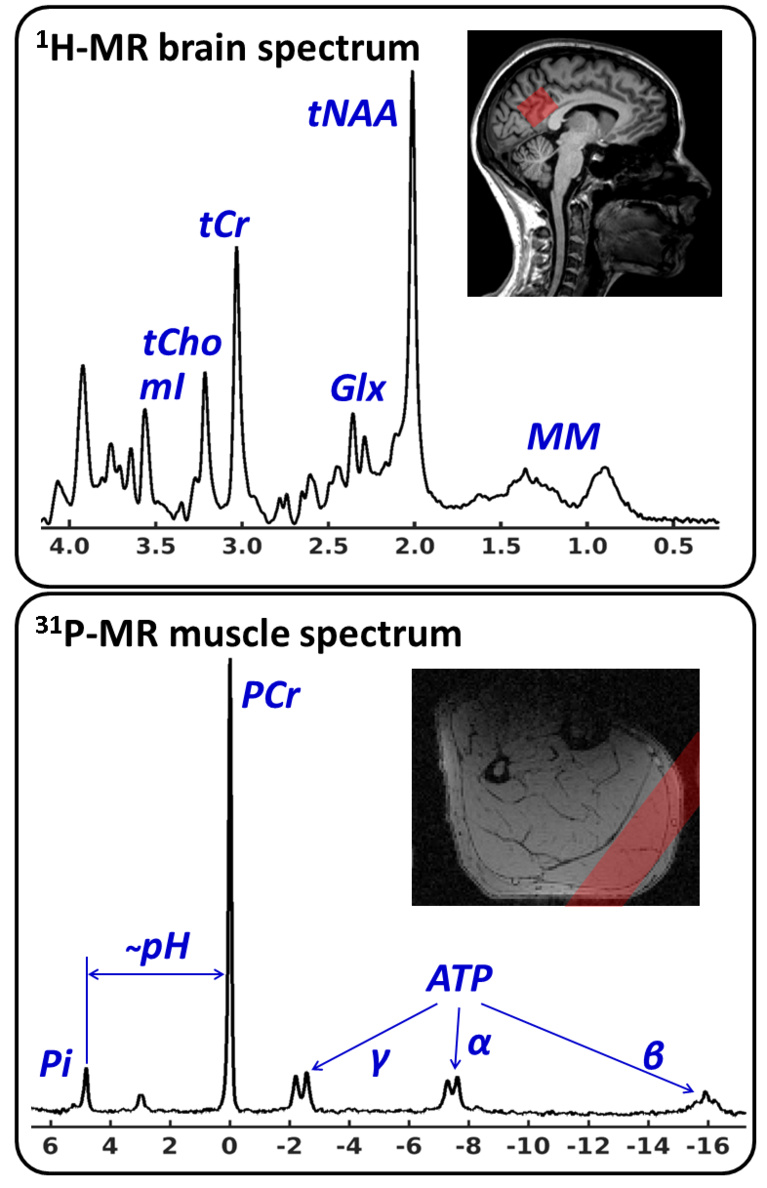
Edited 1H-MR spectroscopy
Regarding the 1H-MRS, our group is focusing on so-called editing methods allowing quantification of low concentration metabolites, whose signals are typically superimposed by other resonances in conventional MR spectra (≤3T). This applies, for example, to the most important brain neurotransmitters GABA and glutamate, which are underlying the regulation of brain function and are therefore important for assessment of neurobiological dysfunctions in psychiatric or neurological diseases (see figure on the left). Besides being focused on development of editing sequences (macromolecule suppression, localization) or appropriate data reconstruction and correction algorithms (phase and frequency correction, subtraction artifact suppression), our group is also supporting clinical and neurophysiological applications of editing methods. For example, functional edited 1H-MRS scheme was implemented and successfully applied to measure acute pain evoked changes of GABA and glutamate to investigate neurometabolic processes underlying the pain processing in healthy brain. Currently, edited 1H-MRS is used to study inhibition-excitation disbalances and accompanied effects on functional connectivity in patients with chronic low back pain. This study is conducted in collaboration with the department of pain medicine on University Hospital Jena and is founded by the German Research Foundation (project GU 1108/03: ‘Does multimodal pain treatment affect neurotransmitter turnover and functional connectivity in the brain of patients with chronic pain?’).
Functional 31P-MR spectroscopy in muscles
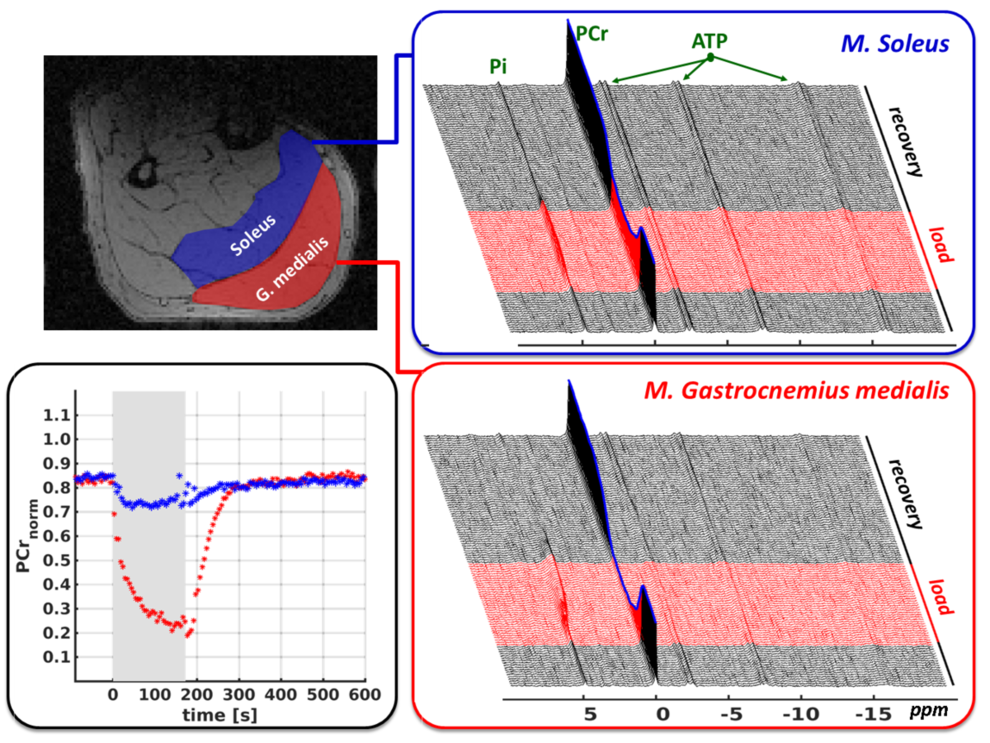
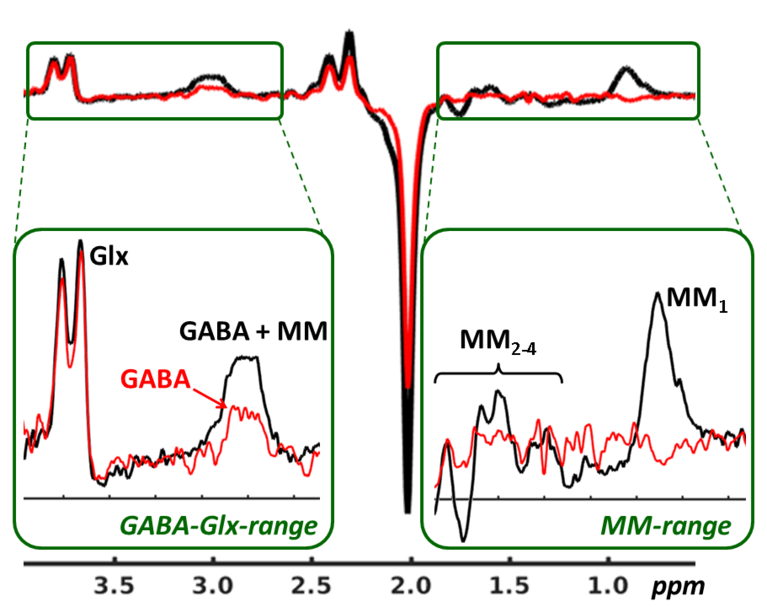
Besides 1H-MRS studies in the brain, the Medical Physics Group is also focusing on functional 31P-MRS measurements of exercise-induced energy metabolism adaptations in human muscles. Acquiring a series of 31P-MR spectra during and after muscle load allows monitoring exercise-induced PCr depletion or PCr recovery during the post-load phase. The initial PCr depletion directly reflects the ATP synthesis rate and immediate ATP demand for given exercise. On the other hand, the post-exercise PCr re-synthesis is a function of mitochondrial ATP production and provides therefore information about the tissue composition of glycolytic and oxidative muscle fibers. Finally, monitoring pH allows assessment of proton accumulation, which is associated with lactate production during anaerobic glycolysis and provides information about processes directly affecting muscle fatigue. Relying on these features, the functional 31P-MRS becomes a powerful tool to examine different metabolic processes non-invasively without any radiation exposure, thus offering wide range of applications in physiology, aging, exercise science, sports medicine and muscle pathology research areas.
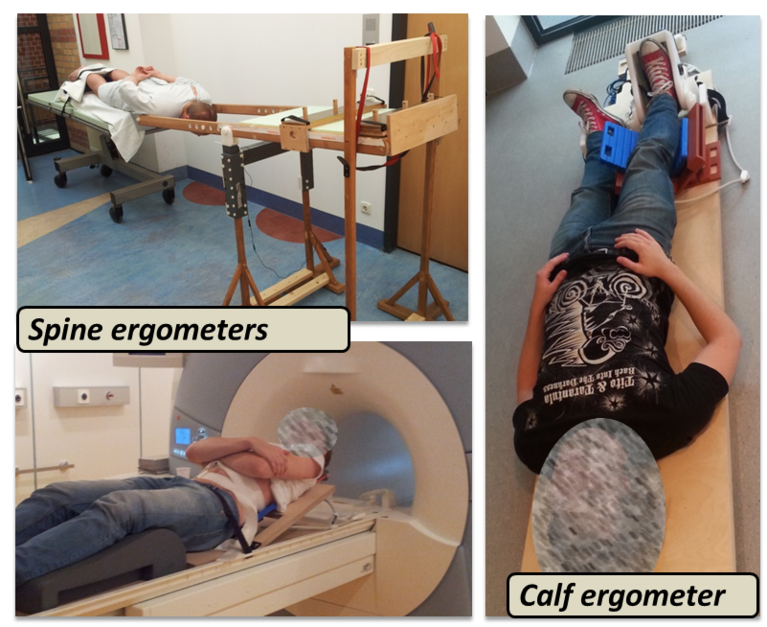
Targeting on time-efficient and reproducible data sampling, our group developed an appropriate 31P-MRS pulse sequence enabling semi-simultaneous and temporally high-resolved measurements of spectral series in multiple muscle groups (see figure on the right). By combining this acquisition technique with global metabolism analytic approaches (spiroergometry and blood lactate diagnostics), it becomes possible to resolve muscle-group specific metabolic adaptations, for example in human calf muscles, and address them to global metabolism adaptations as they are typically applied in exercise physiology studies. Finally, in order to conduct functional 31P-MRS studies in loaded muscles, our group also exhibits a broad experience in setting-up the exercise experiments and developing appropriate MR-compatible ergometers (see figure on left), which allow to apply realistic mechanical loads of defined muscle groups within the MR scanner as well as to monitor the load conditions.
Contact
Publications
Methods, 1H-MRS
M Cleve, M Krämer, A Gussew, JR Reichenbach (2017). Difference optimization: Automatic correction of relative frequency and phase for mean non-edited and edited GABA (1)H MEGA-PRESS spectra. JMR (279), 16–21
M Cleve, A Gussew, G Wagner, K-J Bär, JR Reichenbach (2017). Assessment of intra- and inter-regional interrelations between GABA+, Glx and BOLD during pain perception in the human brain - A combined (1)H fMRS and fMRI study. Neuroscience (365), 125–136
M Cleve, A Gussew, JR Reichenbach (2015). In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional (1)H MEGA-PRESS MR spectroscopy. Neuroimage (105), 67–75
A Gussew, M Erdtel, P Hiepe, R Rzanny, JR Reichenbach (2012). Absolute quantitation of brain metabolites with respect to heterogeneous tissue compositions in (1)H-MR spectroscopic volumes. MAGMA (25), 321–333
A Gussew, R Rzanny, M Erdtel, HC Scholle, WA Kaiser, HJ Mentzel, JR Reichenbach (2010). Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage 49(2): 1895-902.
Methods, 31P-MRS
K Moll, A Gussew, M Nisser, S Derlien, M Krämer, JR Reichenbach (2018). Comparison of metabolic adaptations between endurance- and sprint-trained athletes after an exhaustive exercise in two different calf muscles using a multi-slice 31P-MR spectroscopic sequence. NMR Biomed. 31(4).
K Moll, A Gussew, C Hein, N Stutzig, JR Reichenbach (2017). Combined spiroergometry and (31) P-MRS of human calf muscle during high-intensity exercise. NMR Biomed, e3723
R Rzanny, N Stutzig, P Hiepe, A Gussew, H-A Thorhauer, JR Reichenbach (2016). The reproducibility of different metabolic markers for muscle fiber type distributions investigated by functional (31)P-MRS during dynamic exercise. Z Med Phys (26), 323–338
N Stutzig, R Rzanny, K Moll, A Gussew, JR Reichenbach, T Siebert (2016). The pH heterogeneity in human calf muscle during neuromuscular electrical stimulation. Magnetic Resonance in Medicine
P Hiepe, A Gussew, R Rzanny, C Anders, M Walther, H-C Scholle, JR Reichenbach (2014). Interrelations of muscle functional MRI, diffusion-weighted MRI and (31) P-MRS in exercised lower back muscles. NMR Biomed (27), 958–970
K Tschiesche, M Rothamel, R Rzanny, A Gussew, P Hiepe, JR Reichenbach (2014). MR-compatible pedal ergometer for reproducible exercising of the human calf muscle. Med Eng Phys (36), 933–937
Clinical and physiological applications
S. Smesny, J Große, A Gussew, K Langbein, N Schönfeld, G Wagner, M Valente, JR Reichenbach (2018). Prefrontal glutamatergic emotion regulation is disturbed in cluster B and C personality disorders - A combined 1H/31P-MR spectroscopic study. J Affect Disord. 227: 688-697.
J Steinke, C Gaser, K Langbein, M Dietzek, A Gussew, JR Reichenbach, S Smesny, H Sauer, I Nenadić (2017). Hippocampal metabolism and prefrontal brain structure: A combined 1H-MR spectroscopy, neuropsychological, and voxel-based morphometry (VBM) study. Brain Res., 1677: 14-19.
L Janetzki, A Gussew, R Malessa, U Habenicht, JR Reichenbach, B Strauß, C Borys (2016). Cerebral metabolic changes and chronic back pain : Study taking into consideration clinical and psychological parameters. Schmerz (30), 134-140
G Wagner, A Gussew, S Köhler, F de la Cruz, S Smesny, JR Reichenbach, K-J Bär (2016). Resting state functional connectivity of the hippocampus along the anterior-posterior axis and its association with glutamatergic metabolism. Cortex (81), 104–117
I Nenadic, R Maitra, S Basu, M Dietzek, N Schönfeld, C Lorenz, A Gussew, GP Amminger, P McGorry, JR Reichenbach, H Sauer, C Gaser, S Smesny (2015). Associations of hippocampal metabolism and regional brain grey matter in neuroleptic-naïve ultra-high-risk subjects and first-episode schizophrenia. Eur Neuropsychopharmacol
S Smesny, A Gussew, NJ Biesel, S Schack, M Walther, R Rzanny, B Milleit, C Gaser, T Sobanski, CC Schultz, P Amminger, U-C Hipler, H Sauer, JR Reichenbach (2015). Glutamatergic dysfunction linked to energy and membrane lipid metabolism in frontal and anterior cingulate cortices of never treated first-episode schizophrenia patients. Schizophr Res
G Wagner, M Herbsleb, F Cruz, A Schumann, F Brünner, C Schachtzabel, A Gussew, C Puta, S Smesny, HW Gabriel, JR Reichenbach, K-J Bär (2015). Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab
I Nenadic, M Dietzek, N Schönfeld, C Lorenz, A Gussew, JR Reichenbach, H Sauer, C Gaser, S Smesny (2014). Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophr Res (161), 169–176
I Nenadic, M Dietzek, K Langbein, R Rzanny, A Gussew, JR Reichenbach, H Sauer, S Smesny (2013). Effects of olanzapine on (31) P MRS metabolic markers in schizophrenia. Hum Psychopharmacol (28), 91–93
I Nenadic, M Dietzek, K Langbein, R Rzanny, A Gussew, JR Reichenbach, H Sauer, S Smesny (2013). Superior temporal metabolic changes related to auditory hallucinations: a (31)P-MR spectroscopy study in antipsychotic-free schizophrenia patients. Brain Struct Funct (219), 1869–1872
A Gussew, R Rzanny,D Güllmar, HC Scholle, JR Reichenbach (2011). 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage 54(2): 1315-23